Ivdr Gspr Checklist Template
Ivdr Gspr Checklist Template - We expect that for the majority of products. Web the gsprs are divided into 3 chapters: Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and. Web the checklist will provide an immediate status of the compliance evidence for the ivdr gspr with utilizing the ivdd er information. Web standards, training, testing, assessment and certification | bsi Ce 2797 throughout this guide, our notified body is referenced using its assigned notified body number: They are similar to the. We expect that for the. Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices directive 90/385/eec, and in vitro diagnostic. Tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web standards, training, testing, assessment and certification | bsi They are similar to the. Web the gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with. Please also follow such a structured format when. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and. Tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web a checklist that manufacturers may complete to demonstrate how they have complied with. Web an easier approach to ivdr technical documentation. Web requirements (gspr) within the ivdr. Please also follow such a structured format when. We expect that for the majority of products. Web buy ivdr gspr checklist template * the ivdr gspr checklist template was prepared exclusively on the basis of our expertise and skills by our technical specialists. Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices directive 90/385/eec, and in vitro diagnostic. Checklist ivdr technical documentation (short) download. Web the checklist will provide an immediate status of the compliance evidence for the mdr/ivdr gspr with utilizing the mdd/ivdd er information. We expect that for the. Web an. Checklist ivdr technical documentation (short) download. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and. Web standards, training, testing, assessment and certification | bsi We expect that for the majority of products. Tips, checklists, and templates from seasoned medical device professionals available at your. Web standards, training, testing, assessment and certification | bsi Web the gsprs are divided into 3 chapters: Web an easier approach to ivdr technical documentation. Web the gspr checklist was developed solely on and basis of unsere technical professionals’ knowledge and skills. Web with the implementation of the new eu medical device regulation (mdr 2017/745) and eu in vitro diagnostic. We expect that for the. Sample text has been included in blue. Web buy ivdr gspr checklist template * the ivdr gspr checklist template was prepared exclusively on the basis of our expertise and skills by our technical specialists. Web i'm not sure why you're not able to take your existing essential requirements checklist and paste in the requirements from. Please also follow such a structured format when. Web the checklist will provide an immediate status of the compliance evidence for the mdr/ivdr gspr with utilizing the mdd/ivdd er information. Web how to use this gspr checklist. They are similar to the. Web the gspr is known as general safety and performance requirements are listed in annex i of eu. We expect that for the. Web summary of technical documentation templates. Web the checklist will provide an immediate status of the compliance evidence for the ivdr gspr with utilizing the ivdd er information. Web standards, training, testing, assessment and certification | bsi Page 4 of 10 # requirement standards applied design documentation qualification We expect that for the majority of products. Ce 2797 throughout this guide, our notified body is referenced using its assigned notified body number: Web the checklist will provide an immediate status of the compliance evidence for the mdr/ivdr gspr with utilizing the mdd/ivdd er information. We expect that for the. Please also follow such a structured format when. Web the gspr checklist was developed solely on and basis of unsere technical professionals’ knowledge and skills. Web a checklist that manufacturers may complete to demonstrate how they have complied with the gsprs for an ivd, and where the associated evidence can be found, is available. Page 4 of 10 # requirement standards applied design documentation qualification Tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and. Web how to use this gspr checklist. Web the new eu mdr and eu ivdr, which repealed the medical devices directive 93/42/eec, active implantable medical devices directive 90/385/eec, and in vitro diagnostic. Web standards, training, testing, assessment and certification | bsi We expect that for the. Checklist ivdr technical documentation (short) download. We expect that for the majority of products. Web the gsprs are divided into 3 chapters: Web the gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746. Web i'm not sure why you're not able to take your existing essential requirements checklist and paste in the requirements from the annex of the. Web with the implementation of the new eu medical device regulation (mdr 2017/745) and eu in vitro diagnostic regulation (ivdr 2017/746), the ‘essential requirements’ became. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation. Sample text has been included in blue. Web an easier approach to ivdr technical documentation. Web the checklist will provide an immediate status of the compliance evidence for the mdr/ivdr gspr with utilizing the mdd/ivdd er information. Web the checklist will provide an immediate status of the compliance evidence for the ivdr gspr with utilizing the ivdd er information. Page 4 of 10 # requirement standards applied design documentation qualification Web the checklist will provide an immediate status of the compliance evidence for the ivdr gspr with utilizing the ivdd er information. Tips, checklists, and templates from seasoned medical device professionals available at your fingertips. Web the gspr checklist was developed solely on and basis of unsere technical professionals’ knowledge and skills. Web requirements (gspr) within the ivdr. They are similar to the. Web with the implementation of the new eu medical device regulation (mdr 2017/745) and eu in vitro diagnostic regulation (ivdr 2017/746), the ‘essential requirements’ became. Checklist ivdr technical documentation (short) download. Web the gspr is known as general safety and performance requirements are listed in annex i of eu mdr 2017/745 and eu ivdr 2017/746. Web an easier approach to ivdr technical documentation. Web buy ivdr gspr checklist template * the ivdr gspr checklist template was prepared exclusively on the basis of our expertise and skills by our technical specialists. Web qualitymeddev has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and. Please also follow such a structured format when. Web how to use this gspr checklist. Before placing in vitro diagnostic (ivd) devices on the market, most manufacturers will need their technical documentation. Web the checklist will provide an immediate status of the compliance evidence for the mdr/ivdr gspr with utilizing the mdd/ivdd er information.GSPR checklist
Ivdr Gspr Checklist Template Portal Tutorials
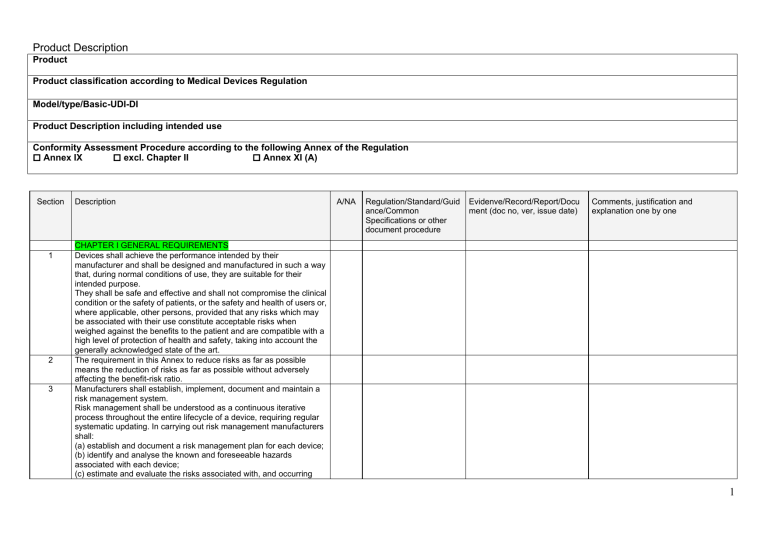
General Safety and Performance Requirements (GSPR) Checklist — Medical
Pin on Help
General Safety and Performance Requirements (GSPR) Checklist — Medical
Mdr Gspr Checklist Template Portal Tutorials
Pin on AC
Comparative table GSPR Essential Requirements (v3) Académie DM Experts
Ivdr Gspr Checklist Template Portal Tutorials
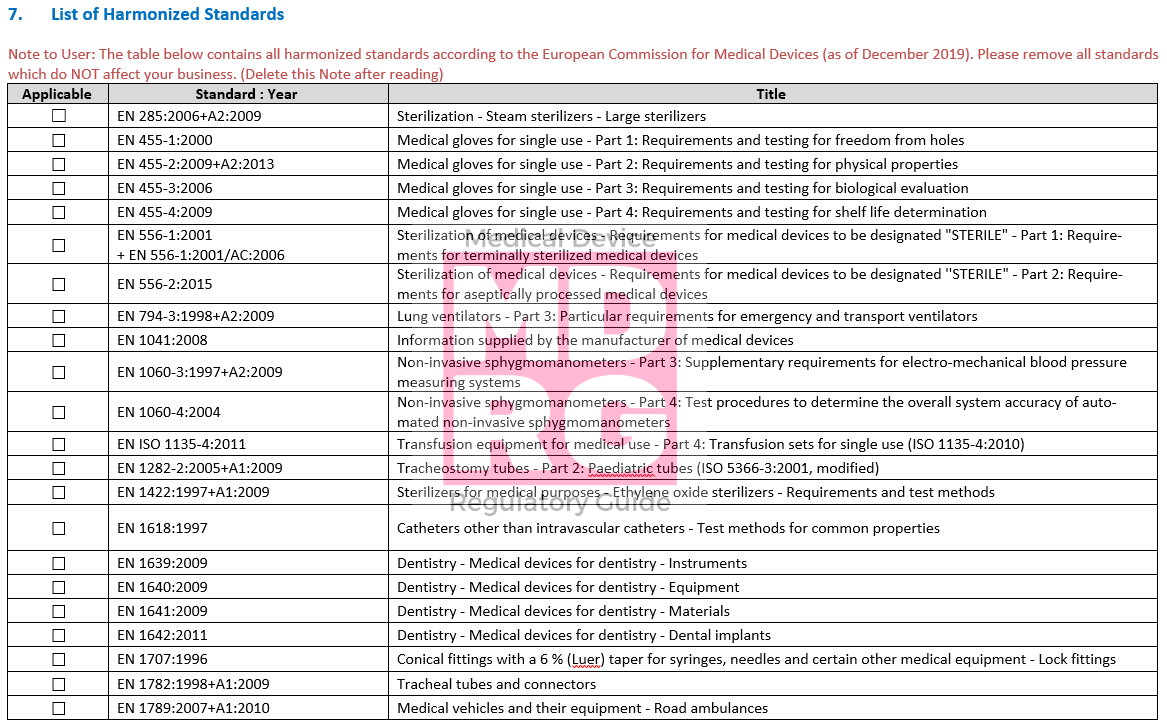
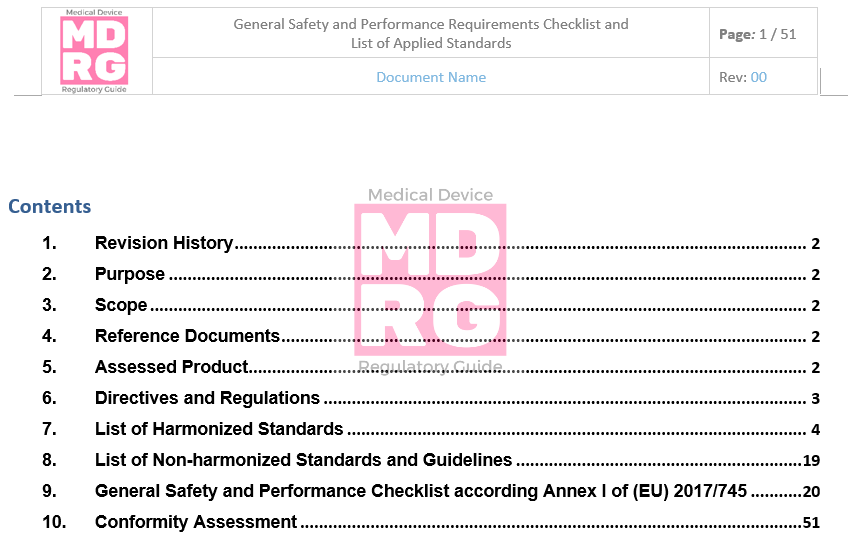
GSPR General Safety And Performance Requirements [EU MDR & IVDR]
Web The New Eu Mdr And Eu Ivdr, Which Repealed The Medical Devices Directive 93/42/Eec, Active Implantable Medical Devices Directive 90/385/Eec, And In Vitro Diagnostic.
Web Standards, Training, Testing, Assessment And Certification | Bsi
Web I'm Not Sure Why You're Not Able To Take Your Existing Essential Requirements Checklist And Paste In The Requirements From The Annex Of The.
We Expect That For The.
Related Post:


+GSPR+Checklist+-+SCREENSHOT+2+WATERMARK.png)

+GSPR+Checklist+-+SCREENSHOT+1+WATERMARK.png)




![GSPR General Safety And Performance Requirements [EU MDR & IVDR]](https://easymedicaldevice.com/wp-content/uploads/2019/11/GSPR-capture.png)