Software Validation Procedure Iso 13485 Template
Software Validation Procedure Iso 13485 Template - Web the paper discusses the challenges of computer systems validation and highlights some validation methods to help medical device companies to comply with fda and iso. Typically, those include slack, github,. Web updated june 8, 2023 iso 13485 templates dr. Web in the latest version of iso 13485, the standard has more explicit requirements for software validation. Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing consistent, accurate and reliable results. Web an iso 13485:2003 certified medical device component manufacturer was preparing for certification on iso 13485:2016. John lafferty has broken down the requirements in the following three elements; How to meet the software validation requirements of iso 13485:2016 2. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web in a nutshell, what does the industry need to do? Web in a nutshell, what does the industry need to do? The documentation template may be used for iso 13485 certification audit purposes. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. John lafferty has broken down the requirements in the following three elements; Web iso 13485 has specifically mandated requirements for process. Web list all your software which you use either in your quality management system or as part of your product development. Web in the latest version of iso 13485, the standard has more explicit requirements for software validation. Web iso 13485 document template: Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. A suggested layout of documenting risk within the master validation plan 3. Typically, those include slack, github,. Web iso 13485 document template: How to meet the software validation requirements of iso 13485:2016 2. Web record of software validation [iso 13485 templates] iso 13485 document template: Web in the latest version of iso 13485, the standard has more explicit requirements for software validation. Web list all your software which you use either in your quality management system or as part of your product development. Web iso 13485 document template: Web iso 13485 has specifically. The standard specifies that any business wanting. Typically, those include slack, github,. John lafferty has broken down the requirements in the following three elements; How to meet the software validation requirements of iso 13485:2016 2. The documentation template may be used for iso 13485 certification audit purposes. Web in the latest version of iso 13485, the standard has more explicit requirements for software validation. The documentation template may be used for iso 13485 certification audit purposes. Web iso 13485 document template: Record of software validation the record provides information about software. Web the paper discusses the challenges of computer systems validation and highlights some validation methods to. How to meet the software validation requirements of iso 13485:2016 2. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web considers to be applicable to the validation of medical device software or the validation of software used to design, develop, or manufacture medical devices. The. Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing consistent, accurate and reliable results. Web iso 13485 document template: Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web considers to be applicable to the validation of medical. Note that the document names in the “fulfilled in document”. Web considers to be applicable to the validation of medical device software or the validation of software used to design, develop, or manufacture medical devices. Web record of software validation [iso 13485 templates] iso 13485 document template: Web software validation and revalidation provides a tool for ensuring any calculations performed. Web in a nutshell, what does the industry need to do? Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing consistent, accurate and reliable results. The standard specifies that. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Web considers to be applicable to the validation of medical device software or the validation of software used to design, develop, or manufacture medical devices. Web list all your software which you use either in your quality management system or as part of your product development. The standard specifies that any business wanting. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Web the paper discusses the challenges of computer systems validation and highlights some validation methods to help medical device companies to comply with fda and iso. Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing consistent, accurate and reliable results. Web record of software validation [iso 13485 templates] iso 13485 document template: Document templates contain an average of twenty comments each, and offer clear. A suggested layout of documenting risk within the master validation plan 3. Web iso 13485 document template: John lafferty has broken down the requirements in the following three elements; Web an iso 13485:2003 certified medical device component manufacturer was preparing for certification on iso 13485:2016. Web updated june 8, 2023 iso 13485 templates dr. Note that the document names in the “fulfilled in document”. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web in a nutshell, what does the industry need to do? Typically, those include slack, github,. The documentation template may be used for iso 13485 certification audit purposes. Record of software validation the record provides information about software. Web software validation and revalidation provides a tool for ensuring any calculations performed via software are producing consistent, accurate and reliable results. Web considers to be applicable to the validation of medical device software or the validation of software used to design, develop, or manufacture medical devices. The standard specifies that any business wanting. Web in a nutshell, what does the industry need to do? Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web record of software validation [iso 13485 templates] iso 13485 document template: Record of software validation the record provides information about software. Web this table maps all requirements of the iso 13485:2016 (by section) to the relevant documents. Document templates contain an average of twenty comments each, and offer clear. A suggested layout of documenting risk within the master validation plan 3. Web an iso 13485:2003 certified medical device component manufacturer was preparing for certification on iso 13485:2016. Web in the latest version of iso 13485, the standard has more explicit requirements for software validation. Web iso 13485 document template: Web list all your software which you use either in your quality management system or as part of your product development. Web iso 13485 has specifically mandated requirements for process validation, for identifying the processes where verification cannot be done, for processes affected by. Note that the document names in the “fulfilled in document”.Design Control Procedures Bundle
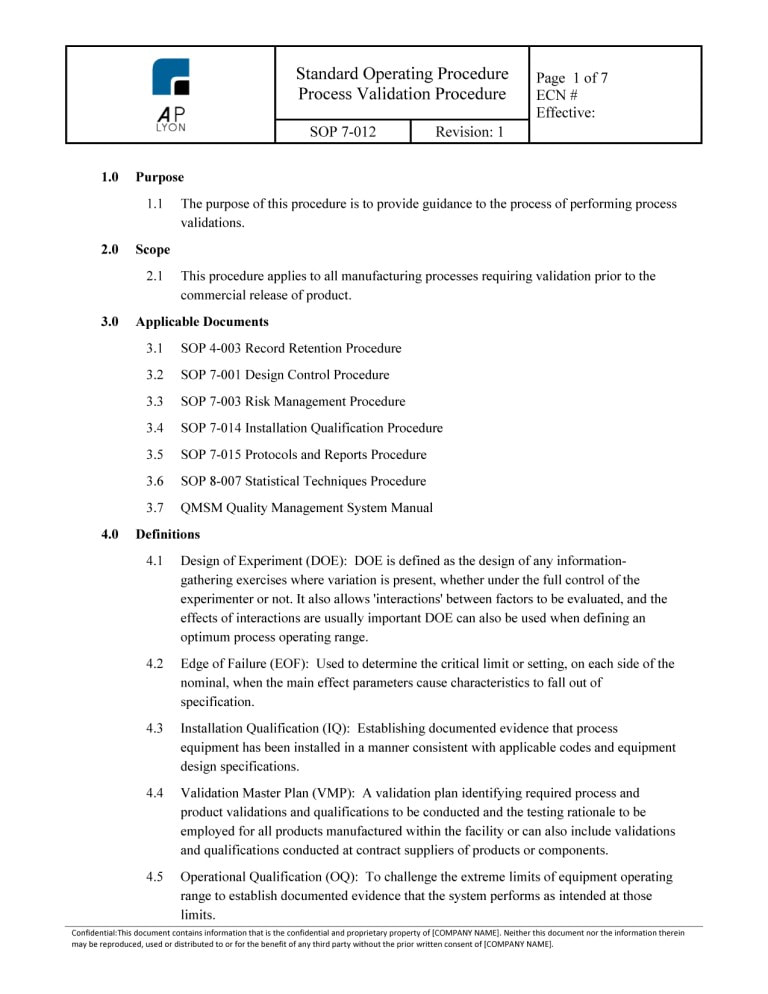
Software Validation Procedure
Software Validation Procedure
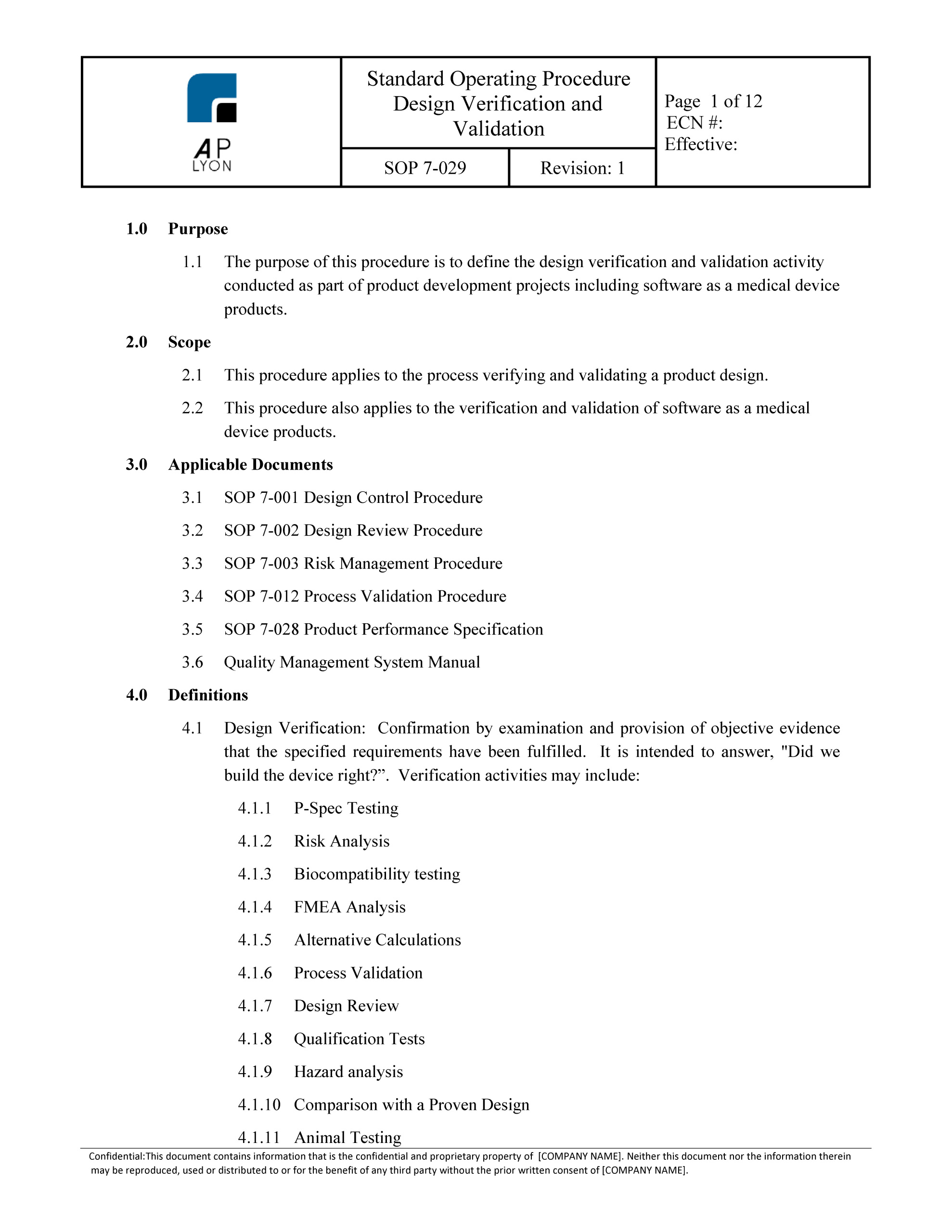
Iso 13485 Software Validation Template PDF Template
ISO 13485 Process Validation Procedure Bundle
Iso 9000 Standard Operating Procedure Template
Medical Device Process Validation Procedure ISO 13485 and FDA QSR
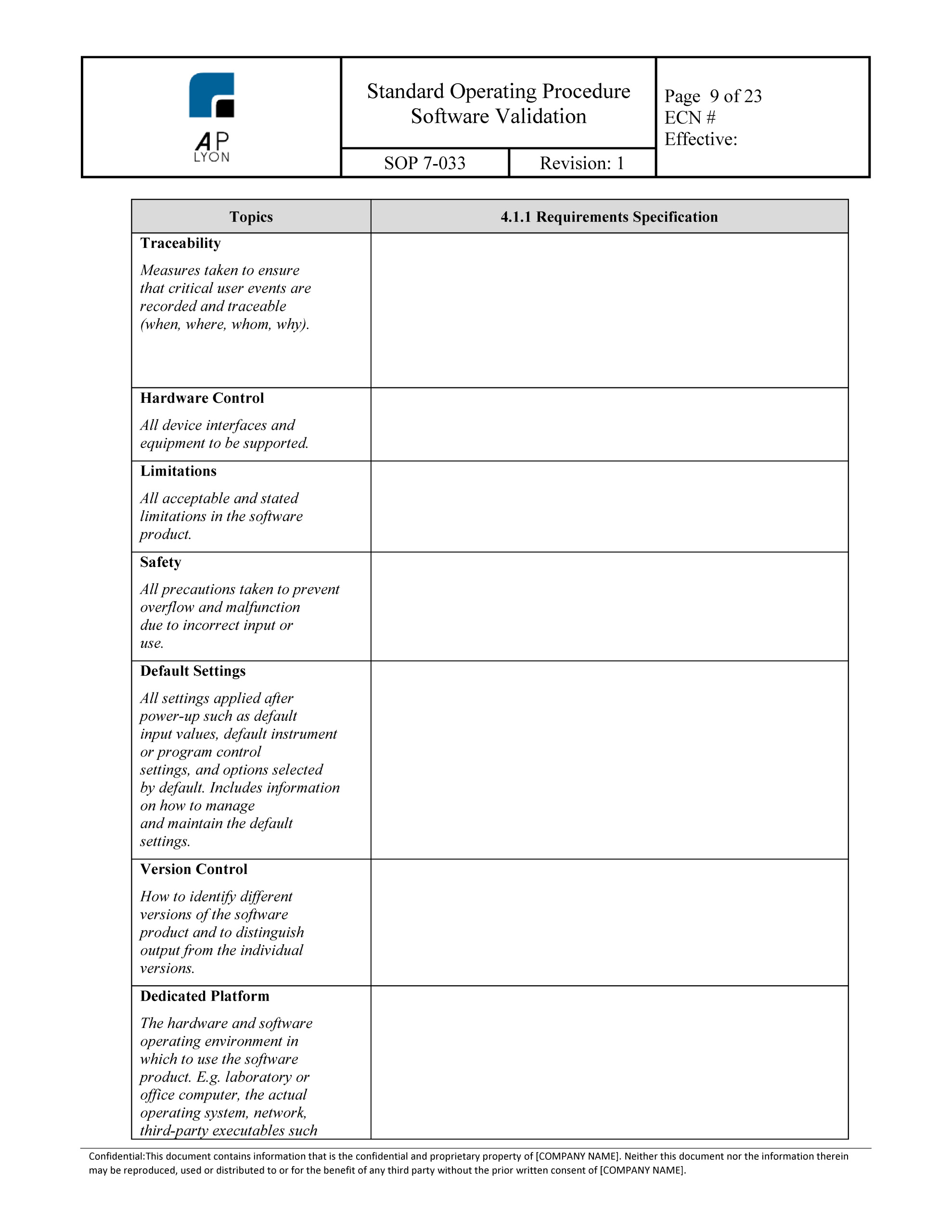
Software Validation Procedure ISO 13485 QMS.4.1.6 template
Validation Master Plan Template Verification And Validation
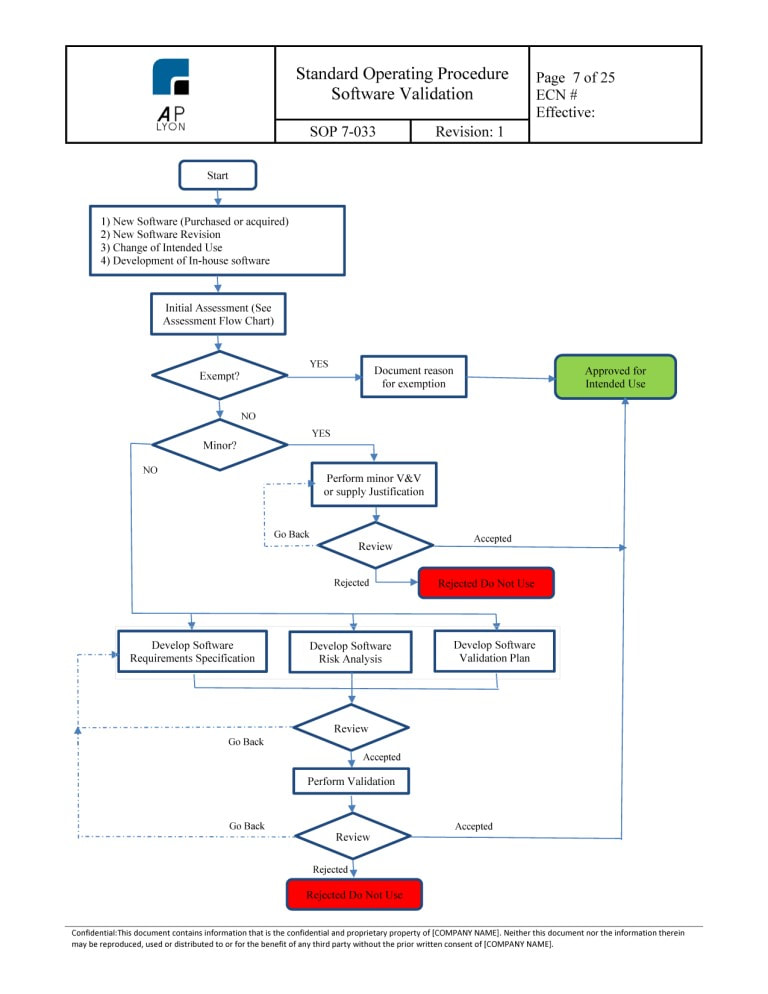
Software Validation Template
Web Updated June 8, 2023 Iso 13485 Templates Dr.
The Documentation Template May Be Used For Iso 13485 Certification Audit Purposes.
Typically, Those Include Slack, Github,.
How To Meet The Software Validation Requirements Of Iso 13485:2016 2.
Related Post: